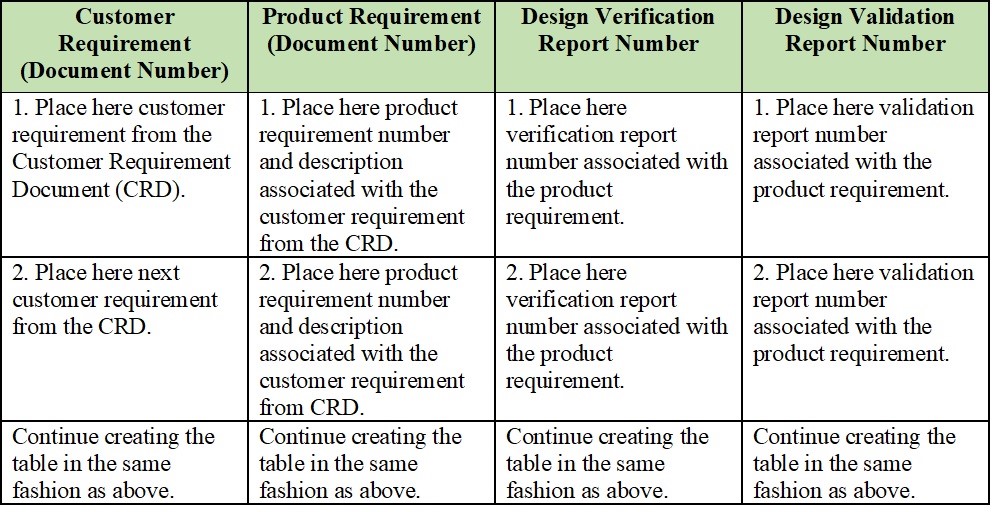
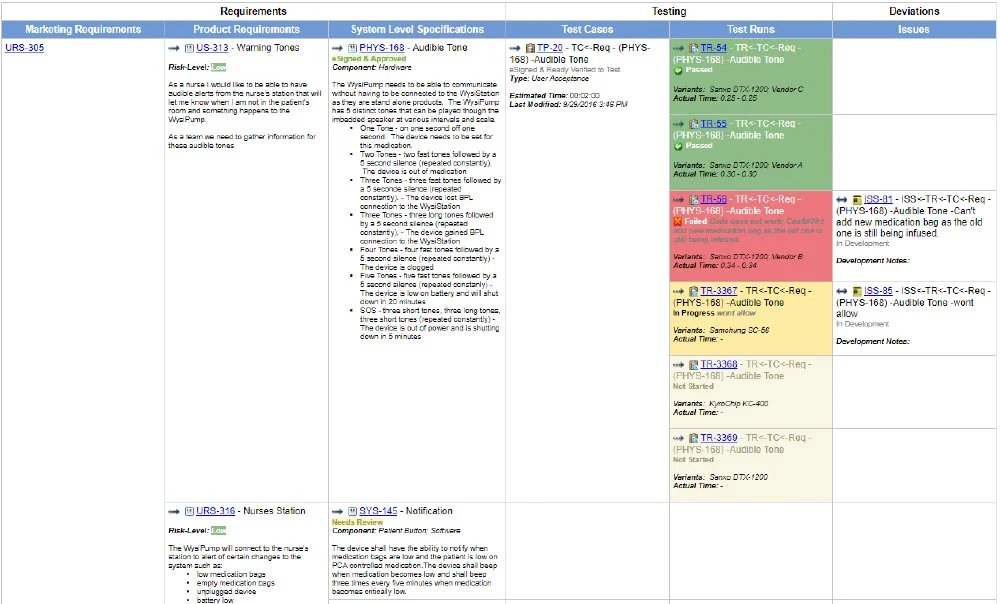
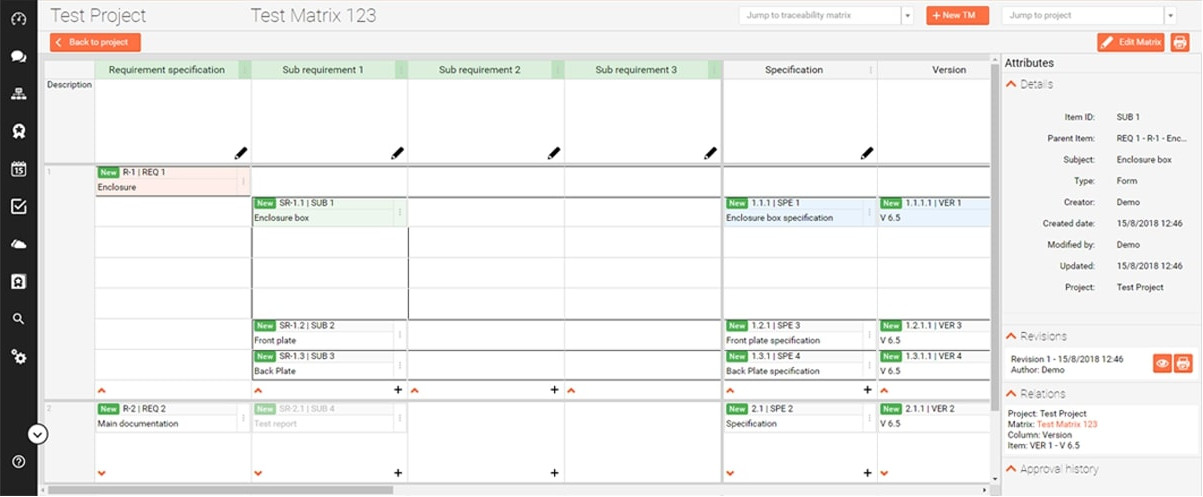
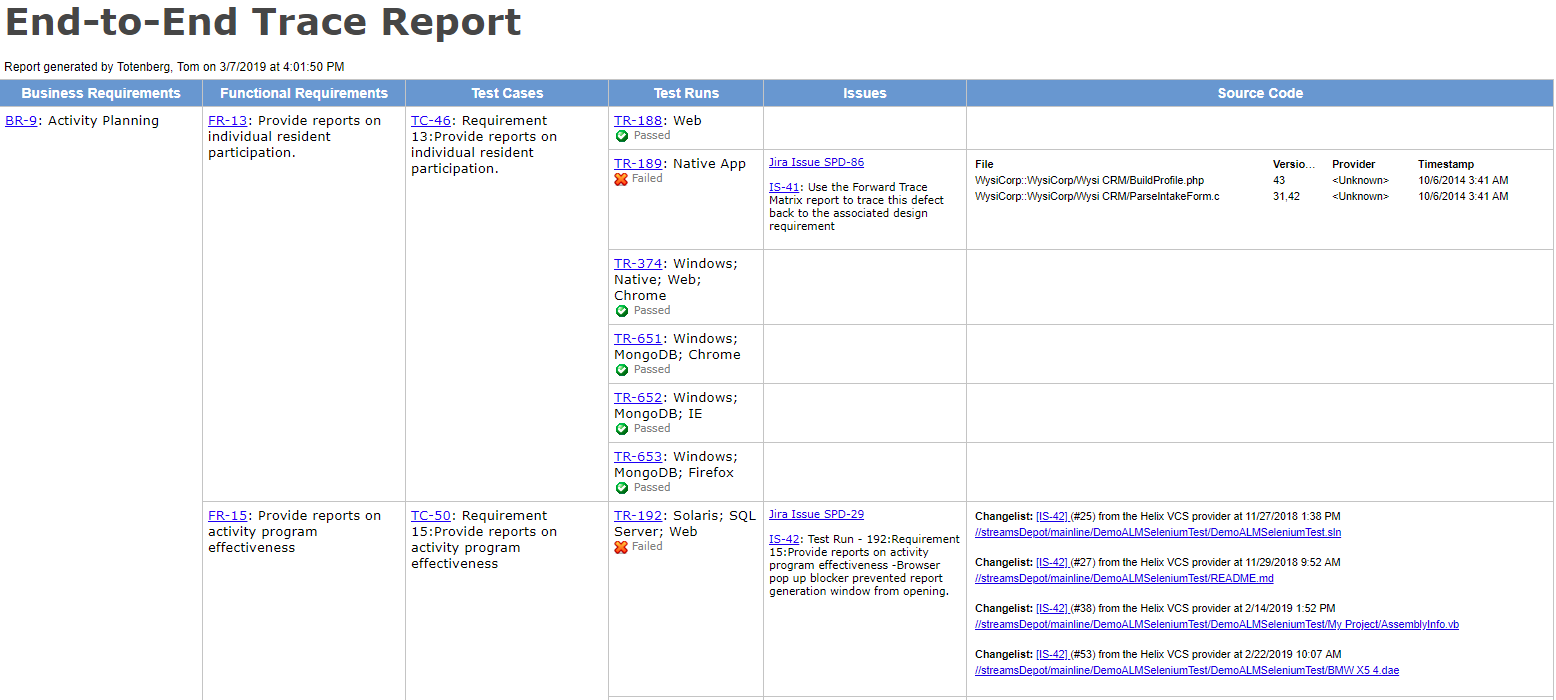
The design requirements traceability matrix drtm is a combination of two documents that have been used for the past two decades by medical device manufacturers.
Medical device design traceability matrix template.
Design validation on the other hand ensures that the device meets user needs and intended uses and will therefore become a viable product in the marketplace.
You ll need to add a column for each of your artifacts.
Laser marking on medical equipment enables you to get permanent marking resistant to sterilization processes.
There are two common types of validation.
1 the design requirements matrix or iovv i e inputs outputs verification and validation and 2 the risk traceability matrix.
Design verification is all about confirming by objective evidence that your device s design output meets its design input so that a manufacturer can say i made the product correctly in most cases comparing outputs to inputs shows that the device.
Is there a documented traceability analysis or matrix linking product design requirements design specifications risks and controls and tests.
Risk analysis hazard traceability matrix template free 0 00 this is a downloadable template which applies to medical devices including in vitro diagnostic medical devices and active implantable medical devices.
For a basic traceability matrix your columns will be.
Traceability of medical devices.
Manufacturers in the medical market and healthcare institutions are regulated by very strict.
This traceability system is able to engrave logos datamatrix codes serial numbers or any other information required to identify a medical device.
Other requirements fda guidance documents.
In this third video in the medical device plm series we show you how you can use minervas medical device plm solution to get a traceability matrix that will.
Risk analysis hazard traceability matrix template free 0 00 design and development plan template medical device per iso 13485 and 21 cfr 820 free 0 00 checklist iso 14971 2007 to iso 14971 2019 free 0 00 status report template full free 0 00 design review record template free 0 00.
Create a traceability matrix template in excel.
Design control guidance for medical device manufacturers 1997.
Confirmation of the device s design.